Isomers are chemical compounds that have the same chemical formula but different properties and atom arrangements. This means they show isomerism and are called isomers.
The term "isomer" comes from the Greek words "isos" (equal) and "meros" (parts). It was first used by Swedish chemist Jacob Berzelius in 1830.
Also Check: Osmosis | Octane Number | Natural resourses |
Related Links: Fossils Fuels
Isomerism Types
There are two main kinds of isomerism, each with different subtypes. These main kinds are Structural Isomerism and Stereoisomerism. The different types of isomers are shown below.
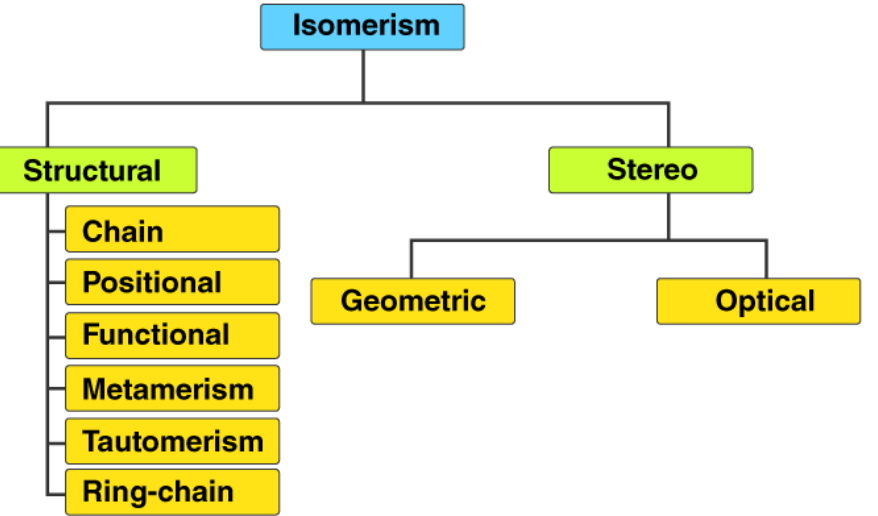
Structural Isomerism
Structural isomers have the same molecular formula but different connectivity of their atoms. There are several types:
Chain Isomerism: Different arrangements of the carbon chain.
Example: Butane (C4H10) can be n-butane or isobutane.
Position Isomerism: Different positions of a functional group on the same carbon chain.
Example: Propanol (C3H8O) can be 1-propanol or 2-propanol.
Functional Group Isomerism: Different functional groups with the same molecular formula.
Example: Ethanol (C2H6O) and dimethyl ether (C2H6O).
Metamerism: Different alkyl groups attached to the same functional group.
Example: Diethyl ether (C4H10O) and methyl propyl ether (C4H10O).
Tautomerism: Rapid interconversion between isomers, typically involving hydrogen shifts.
Example: Keto-enol tautomerism (acetone and prop-2-en-1-ol).
Stereoisomerism
Stereoisomers have the same molecular formula and connectivity but differ in the spatial arrangement of atoms. There are two main types:
Geometric Isomerism (Cis-Trans Isomerism): Different spatial arrangement around a double bond or ring structure.
Example: Cis-2-butene and trans-2-butene (C4H8).
Optical Isomerism: Molecules that are non-superimposable mirror images of each other, often due to the presence of a chiral center (asymmetric carbon).
Example: Lactic acid (C3H6O3) has D-lactic acid and L-lactic acid forms.