Magensium sulphate occurs as kieserite MgSO4.H2O in Stassfurt (Germany) deposit or as Epsom salt in the mineral water of the Epsom springs in England.
Method of Preparation of Epsom salt MgSO4.7H2O
(i) From dolomite
The dolomite ore is boiled with dil. H2SO4.
CaCO3.MgCO3 + 2H2SO4 → CaSO4 ↓ + MgSO4 + 2H2O + 2CO2
The ppt of calcium sulphate are filtered off and the solution on concentration and cooling gives crystals of MgSO4.7H2O.
(ii) From Magnesite
The magensite ore is powdered and dissolved in dilute H2SO4. The resulting solution is concentrated and cooled when crystals of MgSO4.7H2O separate out.
MgCO3 + H2SO4 → MgSO4 + H2O + CO2
(iii) From Kieserite
The mineral Kieserite (MgSO4.H2O) is powdered and dissolved in water. The resulting solution upon concentration and cooling gives crystals of MgSO4.7H2O.
(iv) Laboratory Preparation
In the laboratory MgSO4 is prepared by dissolving Mg metal or MgO or MgCO3 with dilute H2SO4.
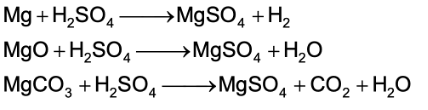
The resulting solution upon concentration and cooling gives crystals of MgSO4.7H2O.
Also Check: Baking Soda
Properties of Epsom salt MgSO4.7H2O
It is deliquescent and readily dissolves in water. Hydrates with 12, 6 and 1 molecule of water of crystallisation are also known. All these hydrates are converted into the anhydrous salt, when heated to 200°C and on further heating they decompose to form the oxide. Magnesium sulphate gives rise to double salt with the alkali sulphate.
(i) Magnesium sulphate is a colourless efflorescent crystalline solid highly soluble in water.
(ii) Isomorphism
MgSO4.7H2O is isomorphous with ZnSO4.7H2O & FeSO4.7H2O compounds having same crystal structure are called isomorphous and the phenomenon is called Isomorphism.
(iii) Action of Heat
When heated it losses 6 molecules of water to give Magnesium sulphate monohydrate which becomes anhydrous when heated to 503 K and finally decomposes to MgO & SO3 gas on strong heating.
![]()
Uses of Epsom salt MgSO4.7H2O
(i) MgSO4 is used as purgative medicine.
(ii) It is used as mordant for cotton in dyeing industry.
(iii) It is used in preparation of fire proof textile and wood.
(iv) Anhydrous MgSO4 is used as a drying agent in organic chemistry.
(v) It is used in preparation of platinised asbestors which is used as a catalyst in the contact process for the manufacture of H2SO4.