What is Electronegativity
It may be defined as the tendency of an atom to attract shared pair of electrons towards itself in a covalently bonded molecules.
The numerical value of the electronegativity of an element depends upon its ionisation potential and electron affinity values. Higher ionization potential and higher electron affinity values implies higher electronegativity value.
Electronegativity Scale
Some arbitrary scales for the quantitative measurement of electronegativities are as under
Pauling's scale: Pauling related the resonance energy(ΔAB) of a molecule AB with the electronegativities of the atoms A and B. If xA and xB are the electronegativities of atoms A and B respectively then
0.208 = √ΔABA – xB if xA>xB or ΔAB = 23.06 (xA – xB)2
= EA-B(experimental) – EA-B(theoretical) where EA-B is the energy of A-B bond. In a purely covalent molecule, AB, the experimental and theoretical values of bond energy A-B are equal.
So ΔAB = 0
or 0=23.06 (xA – xB)2 or xA = xB
In an ionic molecule AB, EA-B(experimental) is more than EA-B(Theoretical).
Pauling assumed the electronegativity value of fluorine 4 and calculated the electronegativity values of other elements from this value.
Mulliken's electronegativity: According to Mulliken, the electronegativity of an element is the average value of its ionisation potential and electron affinity.
or Electro-negativity = Electron affinity + Ionisation potential/2
= Electron affinity + Ionisation potential/5.6 (on pauling scale)
When both are expressed in electron volt
Also Check: Baking Soda | Epsom salt | Glucose | Amino Acids
Related Links: Periodic Table Element | Metal
Factors affecting the magnitude of electronegativity
Atomic radius: As the atomic radius of the element increases the electronegativity value decrease. Electronegativity Α 1/Atomic radius
iii) Effective nuclear charge: The electronegativity value increases as the effective nuclear charge on the atomic nucleus increases.
Electronegativity Α Effective nuclear charge (Zeff)
iii) Oxidation state of the atom: The electronegativity value increases as the oxidation state (i.e. the number of positive charge) of the atom increases.
iv) Hybridisation state of an atom in a molecule: (Reference : Chemical Bonding) If the s- character in the hybridisation state of the atom increases electronegativity increases because s-electrons are comparatively nearer to the nucleus. For example the electronegativity values of C-atom in various hybridisation states are as under:

s-character is increasing
So the electronegativity value is increasing
Periodicity in Electronegativity
i. In a period moving from left to right, the electronegativity increases due to the increase in effective nuclear charge.
ii. In a period the electronegativity value of IA alkali metal is minimum and that of VIIA halogen is maximum.
iii. In a group moving from top to bottom, the electronegativity decreases because atomic radius increases.
iv. Theelectronegativity value of F is maximum and that of Cs is minimum in the periodic table.
v. The electronegativity of Cs(55) should be more than Fr(87) but it is less. This is due to the increase of +32 units in nuclear charge of Fr which makes the effective nuclear charge comparatively high.
vi. On moving from second to third transition series in a group [except Y(39) → La (57)] electronegativity increases due to the increase of +18 units in nuclear charge.
vii. The variation of electronegativity along any period or row of the periodic table may be understood with reference to the following table:
Electronegativity values of some elements in the Pauling scale:
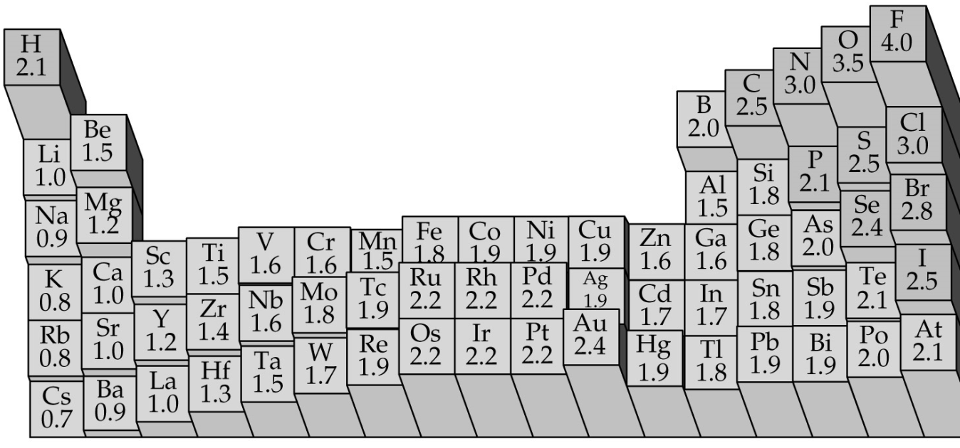
Electronegativity increases form left to right of the periodic table, while it decreases down any group. These observations are consistent with other periodic properties of the atom. The alkali metals possess very feeble attraction for electrons, as it is to be expected from their electronic configurations. The halogens, on the other hand, have highest electronegativity in each row owing to their ns2 np5 configuration. The decrease of the electronegativity down any vertical group in the periodic table is consistent with the variation of effective nuclear charge.
Applications of electronegativity
i) Partial ionic character in covalent Bond: The ionic character of a covalent bond increases as the electronegativity difference of bonded atoms increases. According to Haney and Smith if the electronegativity difference of bonded atoms is Δx then percentage ionic character of the bond = 16Δx+3.5Δx2
If the value of Δx is 2.1 then ionic character percentage is about 50. For example the order of ionic character in H–X bond is as follows–
H–F>H–Cl>H–Br>H–I
Because the electronegativity difference of bonded atoms (Δx) decreases.
ii) Bond strength: If the electronegativity difference of covalently bonded atoms (Δx) increases, the bond energy of the covalent bond also increases. For example – the order of the H–X bond strength is –
H – F > H – Cl> H – Br > H – I
As the bond strength is decreasing the acid strength is increasing. So order of increasing acid strength is
HF <HCl<HBr< HI
iii) Acidic and basic nature of oxides of normal elements in a period: The acidic nature of the oxides of normal elements increases as we move from left to right in a period. In a period from left to right the electronegativity of the elements increases. So the difference of the electronegativities of oxygen and the elements (xO –xE) decreases. If the (xO – xE) values is about 2.3 or more then oxide will be basic. If (xO – xE) values is less than 2.3 the oxide will be acidic. The oxides of the IIIA elements are amphoteric.
The order of acidic or basic nature of the oxides of third period elements may be given as under:
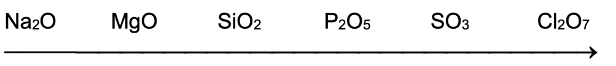
The value of xO – xE is decreasing
Basic nature is decreasing
Acidic nature is increasing
(iv) Metallic and non metallic properties of elements:
i) The metallic character decreases as the electronegativity of the element increases.
ii) On moving from left to right in a period, the electronegativity of the elements increases. So the metallic character decreases.
iii) On moving down a group, the electronegativity of the elements decreases. So the metallic character increases.
v) Basic nature of the hydroxides of elements: A hydroxide MOH of an element M may ionize in two ways in water.
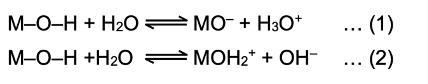
If the ionisation is according to eqn(1) then it is acidic. It is possible when ionic character of O–H bonds is more than the ionic character of M-O bond i.e. (xO – xH) > (xO – xM) where xO, xH and xM are the electronegativities of oxygen, hydrogen and element respectively.
If the ionisation is according to eqn. (2) then it is basic. This is only possible when ionic character of O –H bond is less than M–O bond i.e. (xO –xH) < (xO – xM)
Frequently Asked Questions
Electronegativity is a measure of how much an atom attracts electrons in a chemical bond.
In grade 11, electronegativity is introduced as the ability of an atom to attract electrons towards itself in a chemical bond.
Electronegative elements are those that tend to attract electrons towards themselves in a chemical bond, like fluorine and oxygen.
Electronegative atoms attract electrons in a bond, while electropositive atoms tend to lose electrons and become positively charged.
Fluorine is more electronegative because it has a smaller atomic size and higher nuclear charge, making it attract electrons strongly.
Oxygen (O) has an electronegativity value of approximately 3.44 on the Pauling scale.
Oxygen (O) is more electronegative than carbon (C) because it has a stronger attraction for electrons due to its higher electronegativity value.