Why we need a periodic Table
A periodic table may be defined as a table giving the arrangement of all the known elements according to their properties so that elements with similar properties fall in the same vertical column and elements with dissimilar properties are separated. The periodic table helps us to undergo a systematic study of the various elements found in nature without which it would have been impossible for us to study all the elements. By classifying the elements into various groups and periods a comparative study of the elements and their compounds can be done. It also helps us to analyse the periodic trend in various properties such as ionisation potential, electron affinity, electro negativity etc.
Also Check: Baking Soda | Epsom salt | Glucose | Amino Acids
Discovery of the Periodic table
Dobereiner's Triads, Year of Discovery-1829:
Telluric Helix: Year of Discovery-1862
Newland’s Octet Law, Year of Discovery-1864:
Mendeleev’s Periodic law: Year of Discovery-1869
The long form of Periodic Table or Moseley's Periodic Table- Year of Discovery-1909
(a) Dobereiner's Triads: Although atomic weights were not available for all elements, John Wolfgang Dobereiner in 1829 tried to classify the elements with similar properties in the groups of three elements (Triads). He could succeed in making only a few triads. In the triads of elements the atomic weight of the middle element was the arithmetic mean of the atomic weights of the other two. Some of the triads are as under.

(b) Telluric Helix: It was in 1862, that a periodic classification of the elements was developed that approached the idea we have today. At that time A.E. de Chancourtois,a professor of Geology at the Ecole des Mines in Paris presented an account of his telluric helix in which he indicated the relative properties of elements and their atomic weights.He used a vertical cylinder with 16 equidistant lines on its surface, the lines lying parallel to the axes. Then he drew a helix at 45 degree to the axis and arranged the elements on the spiral in the order of their increasing atomic weights. In this manner, elements that differed from each other in atomic weight by 16 or multiples of 16 fell very nearly on the same vertical line. In addition to the 16 vertical lines, de Chancourtois felt that other connecting lines could be drawn, and that all elements lying on such lines were related in some manner.
(c) Newland’s Octet Law, 1864: Very shortly after the discovery of telluric helix. John Alexander Reina Newland in England made the first attempt to correlate the chemical properties of the elements with their atomic weights. If the elements are arranged in order of their increasing atomic weights, every eighth element had similar properties to first one like the first and eighth note in music. For example,

Mendeleev’s Periodic law: In spite of the importance of the earlier contributions the major portion of the credit for the development of the periodic table must go to the Russian Chemist, DmitriiIvanovich Mendeleev, who proposed
i) The physical and chemical properties of elements are periodic functions of their atomic weights.
ii) If the elements are arranged in the order of their increasing atomic weights, after a regular interval elements with similar properties are repeated.
The long form of the Periodic Table and the Electronic Configuration of elements
Many different forms of a periodic classification of the elements have appeared since the 1871 table by Mendeleev. Each table was designed to point up the various trends and relationship, which its author considered most significant. From the literally hundreds of tables which have been proposed, perhaps the most popular and easily reproduced periodic table is the conventional extended or long form, which is shown in table.
i) Each period starts with an alkali metal whose outermost electronic configuration is ns1.
ii) Each period ends with a noble gas of outermost electronic configuration ns2np6 except He. The electronic configuration of He is 1s2.
iii) The number of elements in a period is equal to the number of necessary electrons to acquire ns2np6 configuration in the outermost shell of first element (alkali metal) of the period. First period contains two elements.
v) The number of elements in each period may be determined by the number of electrons in a stable configuration as under
Groups use in Periodic Table
On the basis of electronic configuration, the elements may be divided into four groups.
i) s-Block elements
a) These are present in the left part of the periodic table.
b) These are group IA and IIA elements.
c) In these elements last electron is filled in the s subshell.
d) Electronic configuration of valence shell is ns1-2 (n = 1 to 7).
ii) p-block elements
a) These are present in right part of the periodic table.
b) These constitute the groups IIIA to VIIA and zero group i.e. group 13 to 18 of the periodic table.
c) The last electron is filled in p subshell of valence shell.
d) The electronic configuration of valence shell is ns2np1-6 (n = 2 to 7).
e) ns2np6is stable noble gas configuration. The electronic configuration of He 1s2.
f) Prior to noble gas group, there are two chemically important groups of non-metals.
These are halogens (group 17) and chalcogens (group 16).
iii) d-Block elements
a) These are present in the middle part of the periodic table (between s & p block elements.
b) These constitute IIIB to VIIB, VIII, IB and IIB i.e, 3 to 12 groups of the periodic table.
c) All are metals.
d) The last electrons fill in (n – 1)dsubshell.
e) The outermost electronic configuration is (n-1)d1-10 ns1-2 (n = 4 to 7).
f) There are three series of d-block elements as under:
3d series – Sc(21) to Zn (30)
4d series – Y (39) to Cd (48)
5d series – La (57), Hf (72) to Hg (80)
iv) ¦-Block elements
a) These are placed separately below the main periodic table.
b) These are mainly related to IIIB i.e. group 3 of the periodic table.
c) There are two series of ¦-block elements as under:
4¦ series – Lanthanides – 14 Elements from Ce (58) to Lu (71)
5¦ series – Actinides – 14 Elements from Th (90) to Lr (103)
d) The last electron fills in (n – 2) ¦ subshell.
e) Their outermost electronic configuration is (n-2)¦1-14 (n-1)s2 (n-1)p6 (n-1)d0-1ns2
(n = 6 and 7).
Representative elements: (s and p-block elements):
They include all the elements:
Group IA: Alkali metals – as they form strong alkalies with water.
Group IIA: Alkaline earth metals- form strong alkalies with water.
Group IIIA: Boron family- boron is the first member of the group.
Group IVA: Carbon family – carbon being the first member.
Group VA: Picogens(means suffocation maker) or Nitrogen family.
Group VIA: Chalcogens(means ore former) or Oxygen family.
Group VIIA: Halogens(means salt former).
Periodic Properties
From the discussion of the periodic table, it is evident that those properties, which depend upon the electron configuration of an atom, will vary periodically with atomic number.The real meaning of the word periodic in any classification of elements is that when the elements are arranged in order of their increasing atomic numbers in the same period or a group, there is a gradual change, (i.e. increase or decrease) in a particular property. Some of the more common properties, which depend upon electronic configurations, are:
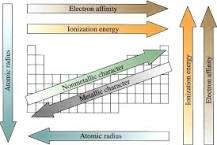
Periodic Properties of the Elements
- Atomic Radius.
- Ionization Energy (ionization potential)
- Electron Affinity.
- Electronegativity.
- Metallic Character.
- Other Trends.
Uses of the periodic table
- It helps in classifying the elements in a systematic manner.
- It helps to calculate the atomic masses of elements.
- It helps to predict the existence of unknown elements.
- Reactivity of elements along the period and down the group can be easily understood.
- There are different areas for metals, non – metals and metalloids.
- The acidic or basic character of the oxides of elements can be figured out from the periodic table.
- Cations are usually formed by metals and anions by non metals can be easily understood.
- The electronic configuration of an element can be easily worked out from the periodic table.
- The valency of an element can be determined from its position in the periodic table.
- The type of bond that the two elements (or two atoms of one element) will form with each other depends upon their position in the periodic table.
periodic law is based on atomic numbers.
Frequently Asked Questions
The first 30 elements include hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, argon, potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, and copper.
The first 60 elements include all those from hydrogen to neodymium, covering hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, argon, potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, gallium, germanium, arsenic, selenium, bromine, krypton, rubidium, strontium, yttrium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, silver, cadmium, indium, tin, antimony, tellurium, iodine, and xenon.
The number 118 reflects the elements that have been discovered and confirmed to exist naturally or artificially. Each element is unique in its atomic structure and properties.
The symbols for the first 20 elements are H (hydrogen), He (helium), Li (lithium), Be (beryllium), B (boron), C (carbon), N (nitrogen), O (oxygen), F (fluorine), Ne (neon), Na (sodium), Mg (magnesium), Al (aluminum), Si (silicon), P (phosphorus), S (sulfur), Cl (chlorine), Ar (argon), K (potassium), and Ca (calcium).
The 118 elements and their symbols include hydrogen (H), helium (He), lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), neon (Ne), sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), argon (Ar), potassium (K), calcium (Ca), and so on up to oganesson (Og).
The first 30 elements with their symbols are H (hydrogen), He (helium), Li (lithium), Be (beryllium), B (boron), C (carbon), N (nitrogen), O (oxygen), F (fluorine), Ne (neon), Na (sodium), Mg (magnesium), Al (aluminum), Si (silicon), P (phosphorus), S (sulfur), Cl (chlorine), Ar (argon), K (potassium), Ca (calcium), Sc (scandium), Ti (titanium), V (vanadium), Cr (chromium), Mn (manganese), Fe (iron), Co (cobalt), Ni (nickel), Cu (copper), and Zn (zinc).
The modern periodic table was developed by Dmitri Mendeleev in 1869.