Osmosis: The movement of solvent or water from higher concentration (solvent) to lower concentration (solvent) through a semipermeable membrane is called as osmosis or the movement of solvent or water from lower concentration to higher concentration of solution through a semipermeable membrane is called as osmosis. Osmosis can also be called as diffusion of solvents”.
- Endosmosis: Movement of solvent into the cell is called as endosmosis.
- Exosmosis: Movement of solvent outside the cell is called as exosmosis.
Also Check: Baking Soda | Epsom salt | Glucose | Amino Acids
Related Links: Periodic Table Element | Metal | Electogravity
Difference between True Solution, Suspe
Types of solution on the basis of concentration:
- Isotonic solution: When the concentration of the solution outside is equal to the concentration of cytoplasm of the cell it is called as isotonic solution.
- Hypertonic solution: When the concentration of the solution outside the cell is more than that of inside the cell. Due to this cell looses water and becomes plasmolysed.
- Hypotonic solution: When the concentration of the solution outside the cell is lesser than that of cytoplasm of cell. Due to this cell swells up and bursts.
What is Reverse Osmosis
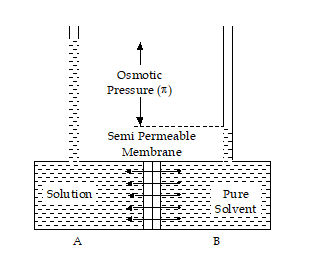
Due to Osmosis, there is net movement of solvent is from the less concentrated (hypotonic) to the more concentrated (hypertonic) solution, which tends to reduce the difference in concentrations. This effect can be countered by increasing the pressure of the hypertonic solution, with respect to the hypotonic.
Thus, Osmotic Pressure (pie) can be defined as the external pressure which must be applied on the high concentration side to maintain equilibrium, and prevent the phenomenon of Osmosis with no net movement of solvent.
If the applied external pressure is more than the osmotic pressure then the solvent particles will start flowing in the opposite direction i.e., solution to solvent side(or high conc. to low conc. Side). This phenomenon is known as reverse osmosis.
Frequently Asked Questions
Osmosis is the movement of water through a membrane from an area with more water to an area with less water.
Osmosis is defined by the movement of water across a semi-permeable membrane.
It’s called osmosis because it involves water moving through a membrane, balancing concentrations.
Osmosis is the movement of water through a membrane; diffusion is the spreading of any particles from high to low concentration.