ADSORPTION ISOTHERM
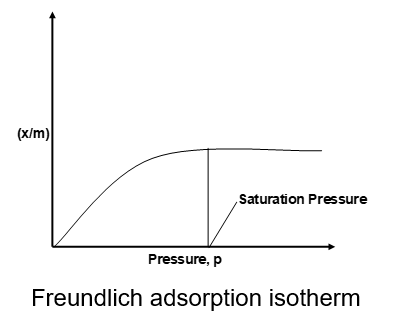
The plots of extent of adsorption (x/m) vs. pressure at constant temperature are called adsorption isotherms. ‘x’ is the amount of adsorbate adsorbed on m gram of adsorbent.
FREUNDLICH’S ADSORPTION ISOTHERM
In case of adsorption of gases on solids, the relation between x/m and the pressure p of the gas at constant temperature is given by the equation.
x/m=Kp1/n(n>1) .....(i)
Freundlich adsorption isotherm
where K and n are the parameter of the equation depending upon the nature of the gas and the solid. According to this, x/m increases with increase of p but since n > 1, x/m does not increase as rapidly as P, as can be seen from the isotherm.
Also Check: Baking Soda | Epsom salt | Glucose | Amino Acids
Related Links: Periodic Table Element | Metal | Electogravity
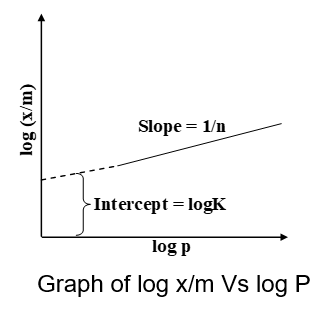
Taking logarithms on both sides of equation (i) we get,
log x/m=logK+1/nlog p .....(ii)
Thus, if we plot a graph between log(x/m) and log p, a straight line is obtained. The slope of the line is equal to 1/n and the intercept on log (x/m) axis will correspond to logK. Therefore, value of K and n can be found out.
LANGMUIR ADSORPTION ISOTHERM
One of the drawbacks of the Freundlich adsorption isotherm is that it fails at high pressure. Langmuir’s adsorption isotherm is based on kinetic theory of gases. Langmuir considered adsorption to consist of the following two opposing processes:
- Adsorption of the gas molecules on the surface of the solid.
- Desorption of the adsorbed molecules from the surface of the solid.
Langmuir believed that eventually a dynamic equilibrium is established between the above two opposing processes. He also assumed that the layer of the adsorbed gas was
unimolecular. Such type of adsorption is obtained in the case of chemisorption, hence isotherm works particularly well for chemisorption.
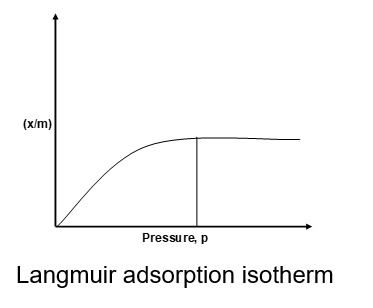
The Langmuir adsorption isotherm is represented by the relation,
x/m=ab/1+bp .....(iii)
Where a and b are two Langmuir parameters. At very high pressure, the above isotherm acquires the limiting from,
x/m=a/b (at very high pressure) bp>>1 .....(iv a)
At very low pressure, equation (iii) is reduced to x/m = ap
(at very low pressure) bp < < 1 …(iv b)
In order to determine the parameters a and b, equation (iii) may be written in its inverse form.
m/x=1+bp/ap=b/a+1/ap .....(v)
A plot of m/x against 1/p gives a straight line with slope and intercept equal to 1/a and b/a, respectively, thus both parameters can be determined.
Langmuir isotherm indicates that at low pressure x/m increases linearly with p. At high pressure, x/m becomes constant, i.e. the surface is fully covered and change in pressure has no effect and no further adsorption takes place, as is evident from figure.
Frequently Asked Questions
Type 3 adsorption isotherm shows adsorption on non-porous solids with weak interactions between adsorbent and adsorbate.
Type 4 adsorption isotherm shows adsorption on porous materials with a capillary condensation in the pores.
Langmuir isotherm describes adsorption on a surface with a finite number of identical sites, while Freundlich isotherm describes adsorption on heterogeneous surfaces.
The best isotherm for adsorption depends on the material and conditions but Langmuir and Freundlich are commonly used.