All matter is made of atoms. Protons and neutrons occupy the centre portion of the atom and the electrons revolve around them in a particular orbit. Electrons which are in the outer most orbit are responsible for conduction of current.
Electrons flow from a body or point where there is an excess of electrons to a body or point where there is a deficit of electrons.
In other words, electrons flow from a body that is negatively charged to a body that is positively charged.
Also Check: Force
Chemical effects of Electric Current
This chapter talks about relation between electric current and metals; and chemical effects thereof.
Conductor: Substances which allow electricity to pass through them are called conductors; such as silver, gold, acidic solution, salt solution, etc.
Insulator: Substances which do not allow electric current to pass through them are called insulators; such as plastic, rubber, etc.
Good conductor and Bad Conductor
Substances which allow electric current to pass through them easily are called good conductors of electricity; such as silver, gold, aluminium, etc.
Some substances allows electric current to pass through them but in very little amount. Therefore, such substances called bad conductors of electricity, rather than being called as insulator.
In fact, most of the substances allow electric current to pass through them under certain conditions, so instead of using terms conductors and insulators, good conductors and bad conductors are used.
Also Check: Tides
Conduction of Electricity
Conduction tester: A conduction tester is a device used to determine whether a substance is a good or poor conductor of electricity.
- Conduction tester using a torch bulb: A simple conduction tester has an electric cell and a torch bulb. One terminal of the cell is connected to one terminal of the bulb by a wire. The other terminal of the cell and bulb have wires which can be brought in contact with materials to test whether they are good or poor conductors of electricity. If the material is a good conductor, the bulb glows and if it is a poor conductor the bulb does not glow.

- Conduction tester using LED: Sometimes the current flowing through the circuit may be too weak and the filament of the bulb may not get sufficiently heated to make it glow. Then instead of a torch bulb an LED can be used in the circuit. LED glows even when a weak current flows in the circuit.
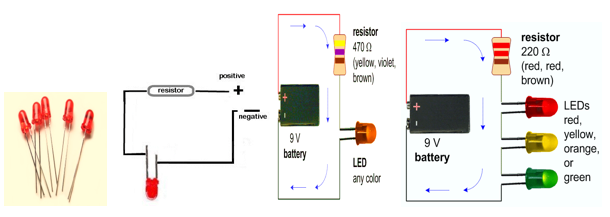
- Conduction tester using a magnetic compass: If a magnetic compass is kept near a wire and current flows through the wire, the magnetic needle gets deflected. So we can use a magnetic compass instead of a torch bulb or LED in the circuit. The magnetic needle gets deflected even if a weak current flows in the circuit.
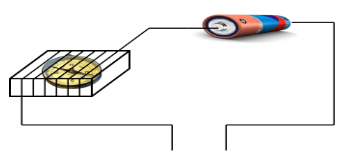
Electrical conductivity of solids
Some solids are good conductors of electricity.
Example:- copper, steel, iron, aluminium etc.
Some solids are poor conductors of electricity.
Example :- wood, plastic, rubber, glass etc
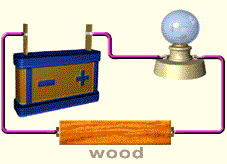
Electrical conductivity of liquids
Do Liquids conduct Electricity?
Some liquids are good conductors of electricity. Example:- tap water, salt solution, hydrochloric acid, sodium hydroxide etc. (solutions of acids, bases and salts are conductors of electricity)
Some liquids are poor conductor of electricity .Example:- distilled water, sugar solution, kerosene, spirit etc.
When we allow electric current to pass through lemon juice or vinegar, both liquids being acidic in nature, allow the current to pass & thus it is proved that liquids that are acidic in nature are good conductors of electricity.
Also Check: Electroscope
It is important to remember that though a material may conduct electricity, it may not conduct it as easily as a metal.
Electric Current produces a magnetic effect. This is proved when the deflection of the magnetic needle is seen when the magnetic needle is kept near a wire & current is flown in it.
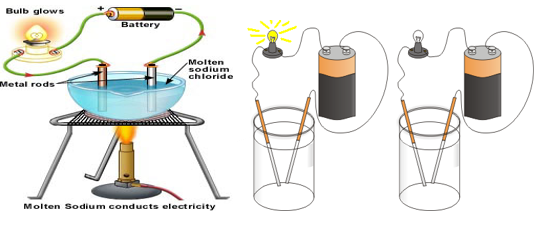
Salt Solutions as Conductors of Electricity
Salt Solutions are good conductors of electricity. When we pass electric current through distilled water, it does not conduct electricity, so it is a poor conductor of electricity.
However, when we add salt to distilled water & conduct electricity, it conducts electricity. This proves that salt solutions are good conductors of electricity.
Tap water, water from lakes, ponds etc, all have salts dissolved in it. So, all these are good conductors of electricity.
Testing of conduction of electricity through liquid
Liquids; which contain salt dissolved in them; conduct electricity. The conduction of electricity through a liquid can be tested using two electrodes and a bulb.
But the conduction of electricity through the liquids which are poor conductor cannot be tested. But conduction of electricity through such liquids can be tested using LED or magnetic compass needle.
Also Check: Buoyant Force
For this, you need a compass needle, an empty matchbox, two electric cells, some wires, a plastic bottle cap and the liquid which is to be tested. Keep the compass needle inside the empty tray of matchbox and wrap a couple of rubber bands around it. Connect the wires to the battery and insert two wires in the liquid which is kept in the plastic bottle cap. You will observe that when the current flows through the wire, there is deflection in the compass needle. This shows that the given liquid conducts electricity. In fact, magnetic compass needle can detect even feeble current. LED can also be used in place of compass needle.

Salts; dissolved in water are responsible for conduction of electricity through water. Tap water, acid solutions, basic solutions, etc. are good conductors of electricity. Distilled water is poor conductor of electricity because in no salt is present in distilled water.
Chemical effects of electric current
When electric current is passed through a conducting solution, some chemical reaction takes place.
Example: -
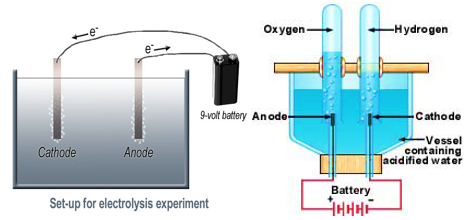
- When electric current is passed through water, water dissociates into hydrogen and oxygen. Hydrogen is deposited over negative pole and oxygen is deposited over positive pole. Deposition of hydrogen and oxygen at different poles is visible in the form of bubbles.
- When electric current is passed through the solution of a metal salt, such as solution of copper sulphate, metal gets deposited at the negative pole, because metal is positively charged.
- Sometimes, the colour of solution also changes when electric current passes through it.
The above examples are some of the chemical effects of electric current. The chemical reaction depends upon the type of solution through which electric current is passed.
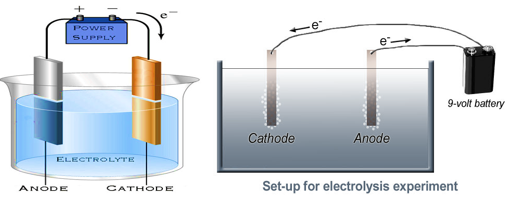
Electrolysis of water
When electric current is passed through water, it splits up into hydrogen and oxygen. This is called electrolysis of water.
Also Check: Thrust & Pressure
When electric current is passed through water oxygen gas bubbles are produced at the electrode connected to the positive terminal of the battery and hydrogen gas bubbles are produce at the electrode connected to the negative terminal of the battery.
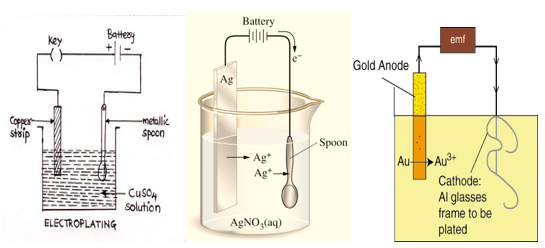
Electroplating
Electroplating is one of the chemical effects of electric current. Electroplating is a chemical process using which a metal is coated with a layer of another desired metal. Electroplating is done to make the metals shiny. Electroplating is done over the articles made of iron to make the iron shiny and to prevent iron from getting rusted.
Example
Wheel rims, handle of cycle, etc. are made shiny by the method of electroplating.
Process of electroplating
In the process of electroplating, metal salt solution is taken in a container. Salt of metal is chosen which is to be coated over another metal. Metal which is to be coated is dipped in the solution and connected with negative pole. Metal for desired coating is connected with positive pole.
When electric current is passed through the solution, metal from anode is dissolved in the salt solution and deposited over the negative pole (cathode). Thus, coating of desired metal is obtained on another metal.
Also Check: Waves
Example:- For electroplating of copper on an object, the object is dipped in acidified copper sulphate solution and connected to the negative terminal of a battery. A copper strip is dipped in the solution and connected to the positive terminal of the battery. When electric current is passed through the solution, copper from the copper strip is deposited on the object.
Use / Benefits of Electroplating:
- Water pipes, which are made of iron, are coated with a layer of zinc metal by the process of electroplating. Zinc is less reactive than iron and thus prevents the iron pipe from getting rusted.
- Rims of wheel of cycle, cars, etc. are electroplated with chromium metal. Layer of chromium metal give them shiny appearance and prevents from rust.
- Ornaments made of silver or other cheap metals are electroplated with gold to give them appearance like gold.
- Tin cans used for storing food are made of iron electroplating with a coat of tin. Tin is less reactive than iron, and prevent foods packed in them from getting spoiled.
Also Check: Sound


Frequently Asked Questions
You can make pure water conduct electricity by adding salts to it.
Saltwater contains more salts than drinking water. This makes saltwater a better conductor of electricity, which creates a stronger magnetic effect, causing the compass needle to deflect more.
Electrolysis involves using an electric current to break down chemical compounds. Electroplating uses an electric current to deposit a layer of metal from a solution onto an electrode.
The water in fire hoses can conduct electricity because it is not pure. Turning off the electricity helps protect firefighters and others from electrocution.
Rainwater, like distilled water, does not naturally conduct electricity well because it is nearly pure. However, when it mixes with air pollutants like sulfur dioxide and nitrogen oxides, it becomes slightly acidic and can conduct electricity, which is why a compass needle may show deflection in rainwater.
Related Links
| S.no | Formulas List |
|---|---|
| 1. | Force |
| 2. | Frictional Force |
| 3. | Thrust and Pressure |
| 4. | Buoyant Force |
| 5. | Waves |
| 6. | Sound |
| 7. | Some Natural Phenomena |
| 8. | Electroscope |
| 9. | Lightning |
| 10. | Earthquake |