How do Acids react with metals?
Let us understand how acids react with metals with the help of an experiment.
Things needed:
- A stand
- A container
- A test tube
- A delivery tube
- A candle
- Dilute sulphuric acid
- Zinc granules
- Soap solution
Procedure:
- Take a
- With the help of the clamp, place the test tube on the
- In this test tube, add about 5 ml of dilute sulphuric
- Then add a few pieces of zinc granules to it. Some gas comes from the surface of zinc granules and also bubbles are
- On the right of the stand, place some soap solution in a Using the delivery tube, pass the gas through the soap solution. Bubbles form in the soap solution and some gas is released.
- Bring a lighted candle near the gas, it will burn with a pop
- A similar reaction can be seen when the sulphuric acid is replaced with other acids like hydrochloric acid or nitric
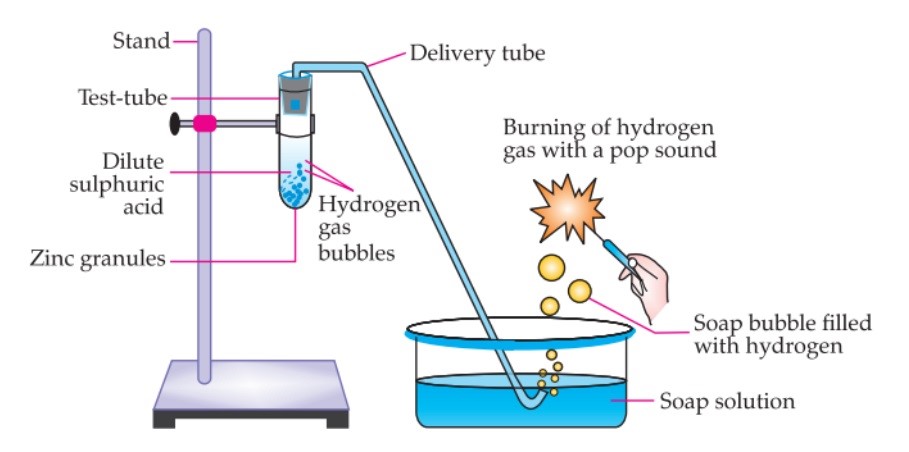
Reaction of Zn granules with dilute sulphuric acid
Observation:
- When a lighted candle is brought near the gas, it burns with a pop
Conclusion:
- The characteristic test for hydrogen gas is burning with a pop sound. This confirms the evolution of hydrogen
In the case of hydrochloric acid, the reaction that takes place in the above experiment is
𝒁𝒏 + 𝟐𝑯𝑪𝒍 → 𝒁𝒏𝑪𝒍𝟐 + 𝑯𝟐
Hydrogen gas and zinc chloride are formed when hydrochloric acid reacts with zinc metal.
Zinc, being more active, displaces the hydrogen from the acid to give zinc chloride which is a salt. Thus, acids react with metals to give us salt and hydrogen gas.
This can be generalized as
Acid + Metal → Salt + Hydrogen Gas
How do Bases react with metals?
When an alkali or a base reacts with metal, it produces salt and hydrogen gas, like acids. This can be generalized as
Alkali (Base) + Metal → Salt + Hydrogen Gas
For example, sodium hydroxide reacts with zinc metal to give us sodium zincate and hydrogen gas.
𝟐𝑵𝒂𝑶𝑯 + 𝒁𝒏𝑵𝒂𝟐 → 𝒁𝒏𝑶𝟐 + 𝑯𝟐
