Share
(i) Sodium oxide
(ii) Aluminum chloride
(iii) Sodium sulphide
(iv) Magnesium hydroxide
ReportQuestion
(i) The chemical formula of a compound is a symbolic representation of its composition. The combining power or combining capacity of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. Some elements show more than one valency.
While writing the chemical formulae for compounds, we write the constituent elements or their symbols and their valencies as shown below. Then we must crossover the valencies of the combining atoms. The positive and negative charges must balance each other and the overall structure must be neutral.
Formula of sodium oxide can be written as follows:
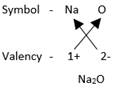
In sodium oxide, there are two sodium ions for each oxide ion. Thus, the formula for Sodium Oxide is Na2O.
(ii) The chemical formula of a compound is a symbolic representation of its composition. The combining power or combining capacity of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. Some elements show more than one valency.
While writing the chemical formulae for compounds, we write the constituent elements or their symbols and their valencies as shown below. Then we must crossover the valencies of the combining atoms. The positive and negative charges must balance each other and the overall structure must be neutral.
Formula of Aluminum chloride (made of Aluminum Al and chlorine Cl):
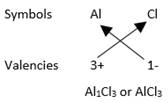
In Aluminium Chloride, there are three chloride ions per one aluminium ion. Thus, the Aluminium chloride is AlCl3.
(iii) The chemical formula of a compound is a symbolic representation of its composition. The combining power or combining capacity of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. Some elements show more than one valency.
While writing the chemical formulae for compounds, we write the constituent elements or their symbols and their valencies as shown below. Then we must crossover the valencies of the combining atoms. The positive and negative charges must balance each other and the overall structure must be neutral.
.jpg)
In sodium sulphide there are two sulphide ions per sodium ions. Therefore, the formula of sodium sulphide is, Na2S.
(iv) The chemical formula of a compound is a symbolic representation of its composition. The combining power or combining capacity of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. Some elements show more than one valency.
While writing the chemical formulae for compounds, we write the constituent elements or their symbols and their valencies as shown below. Then we must crossover the valencies of the combining atoms. The positive and negative charges must balance each other and the overall structure must be neutral.
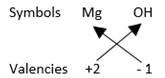
In magnesium oxide, there are two magnesium ions per hydroxide ion. Therefore, the
formula or Sodium Oxide is Mg(OH)2
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply