Share
Write chemical equations for the following conversions:
(i) Nitrobenzene to benzoic acid.
(ii) Benzyl chloride to 2 - phenylethanamine.
(iii) Aniline to benzyl alcohol.
ReportQuestion
Explanation:
(1)
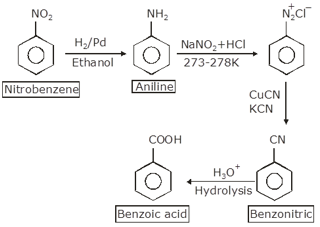
In the first step nitrobenzene is converted to aniline by reduction with h2/Pd.
Then aniline is converted to diazonium salt with and this salt is reacted with copper cyanide to form benzonitrile (Sandmeyer reaction). Benzonitrile on hydrolysis gives benzoic acid.
(2)Benzyl chloride to 2-phenylethylamine
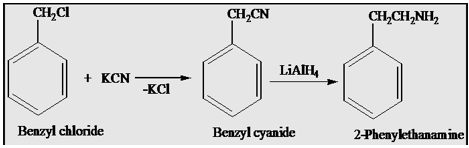
The first steps involved attack of Cyanide Ion on the benzyl chloride to give Benzyl cyanide then benzyl cyanide cyanide on reduction with Lithium aluminum hydride gives 2-phenylethylamine.
(3) Aniline to Benzyl alcohol.
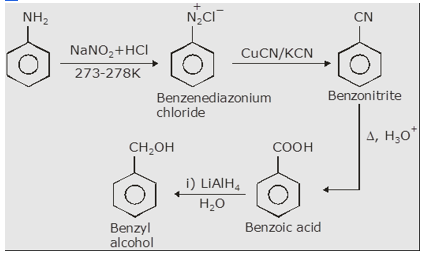
We have discussed the conversion of aniline to benzoic acid in question (1). Now the benzoic acid formed on reduction with Lithium aluminum hydride form Benzyl alcohol.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply