Share
Question
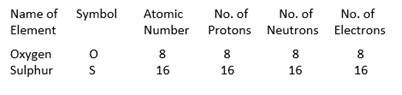
The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom.
(i) Mass number of oxygen = No. of protons + No. of neutrons = 8 + 8 = 16
Mass number of Oxygen is 16
(ii) Mass number of sulphur = No. of protons + No. of neutrons = 16 + 16 = 32
Mass number of sulphur is 32
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply