Share
Report
Question
Explanation:
In an electrolytic cell, the cathode is considered to be negative and the anode is positive because cathode is an electrode where reduction takes place, therefore cathode acts as an electron acceptor. So at cathode the electron master is transferred to force the reduction therefore the cathode becomes negative. But in anode oxidation takes place, so it removes electrons and becomes positive.
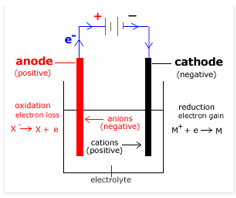
Final answer:
In electrolytic cells, electrical energy causes electrolysis of compounds, leading to chemical change. So, in this cell the battery pumps electrons from anode to cathode. The electrons move away from anode making it positive and electron reaches to cathode, making it a negative terminal.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply