Share
Report
Question
Explanation:
Alkyl amines are stronger bases than aryl amines as lone pair of electrons on N in aromatic amine is delocalised due to resonance with benzene ring.
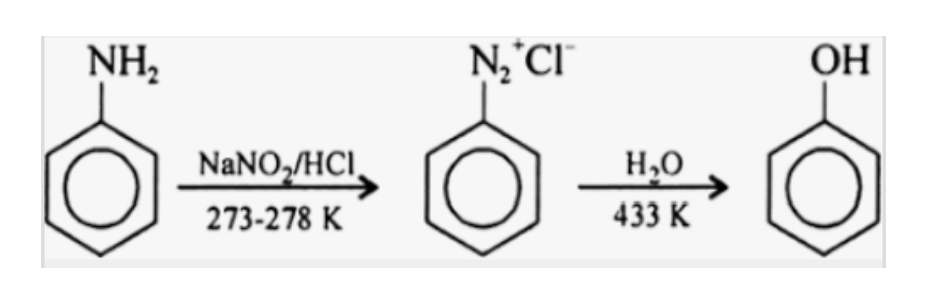
Aryl amines react with nitrous acid to produce phenols.
Alkyl amines are stronger bases than ammonia as +I group provided from alkyl group due to which alkyl amine readily donate it’s electron pair.
Final Answer:
Hence, Option (B) is correct.
Alkyl amines react with nitrous acid to produce unstable diazonium salts.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply