Share
What is the concentration of sugar (C12H22O11) in mol L-1 if its 20 g are dissolved in enough water to make a final volume up to 2L?
ReportQuestion
Explanation:
Given weight of sugar (C12H22O11) = 20g
Molar mass of sugar = 12x mass of carbon+22x mass of hydrogen + 11xmass of oxygen
Molar mass of sugar =12 x 12 + 22 x 1 + 11 x 16
So molar mass = 342 gml-1
Number of moles of sugar =
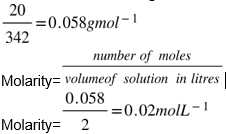
Final Answer:
concentration of sugar will be l0.02ml-1
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply