Share
Report
Question
CN has 13 electrons
Electronic configuration
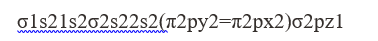
Bond order = 12[B-A]
B = no. of electron in bonding orbital
A = no. of electron in anti bonding orbital
In CN B = 9
A = 4
Bond order = 12[9-4] = 2.5
Bond order = 2.5 x 4 = 10
Hence the correct answer is bond order = 10
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply