Share
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand
ReportQuestion
Explanation:-
Spectrochemical series is basically a list of ligands in which the ligands are arranged in increasing order on the basis of their ligand field strength.
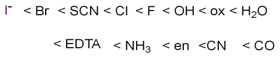
Weak field ligands:
Weak field ligands are those which lies on the left side in spectrochemical series. They cause less splitting of d-orbitals due to which no pairing of electrons occurred in the crystal field splitting diagram.
Examples: F-1, c1-1
Strong field ligands:
Strong field ligands lies on the right side of the spectrochemical series.
They cause large splitting of d-orbitals and forced the electrons to pair up in the crystal filled splitting.
Examples -CO, cyanide ion
Final Answer-
In spectrochemical series ligands are arranged in increasing order of their ligand field strength and those lies on the left side are called weak field ligands as they are not able to pair up the electrons in CFT diagram.
Ligands lies on the right side of the series are called strong field as they are able to force the electrons to pair up in the CFT diagram.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply