Share
Report
Question
Explanation: The metal reactivity series is a list in which metals are grouped in decreasing order of their chemical reactivity.
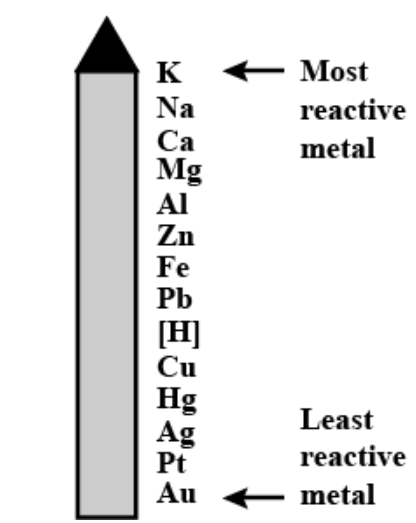
Its importance are:
- From potassium to gold, the ease with which a metal in solution loses an electron(s) and forms a positive ion decreases.
- Hydrogen is included in the activity series because, like metals, it loses an electron in most chemical reactions and becomes positively charged (H+).
Final answer: The metal reactivity series is a list in which metals are grouped in decreasing order of their chemical reactivity.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply