Share
Report
Question
It is a hypothetical concept and comes under valence bond theory.
In it atomic orbitals of almost similar energy and proper symmetry are intermixed together to form a new hybrid orbitals for better Bond formation and minimum repulsion.
The number of new hybrid orbitals is equal to the number of atomic orbits intermixed together.
sp orbitals are born by mixing of one s and one p-orbital and their shape is linear.
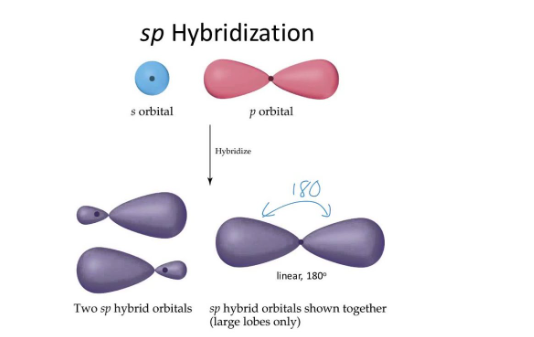
sp2 Orbitals are formed by mixing of one s and two p-orbital and their shape is trigonal planar.
Sp3 Orbitals are formed by mixing of one s and three p-orbital and their shape is tetrahedral.
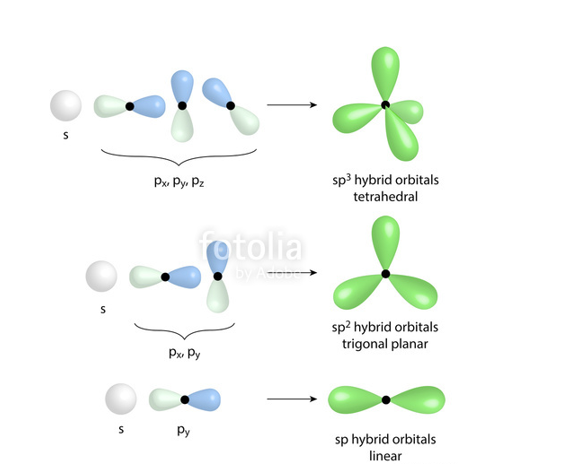
Hybridization ismixing of atomic orbitals to form new hybrid orbitals and the shape of sp, sp2 and sp2 linear ,trigonal planar and tetrahedral
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply