Share
Report
Question
Electromeric effect is one of the temporary effects that is shown by organic molecules having multiple bonds.
This effect is shown by the molecule in the presence of some attacking reagent.
In the presence of an attacking reagent, complete transfer of shared takes place from one atom to another and an instantaneous dipole is formed.
Electromeric effect is of two types:
+E effect
- E effect
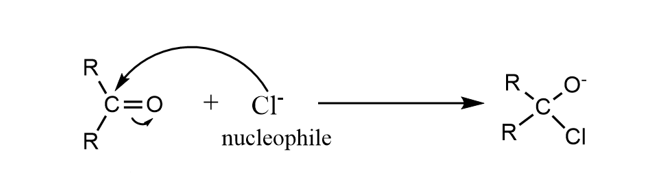
+E effect is observed when the attacking reagent is an electrophile and -E is observed when the attacking reagent is a nucleophile.
<img alt="chemistry figure" class="img-reponsive" loading="lazy" title="" chemistry="" figure"="" data-cke-saved-src="https://apiengine3.home-tution.com/webroot//files/8w.png" src="https://apiengine3.home-tution.com/webroot//files/8w.png">
In the +E effect, the shared is shifted or moved towards the attacking reagent, as shown in the example below:
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply