Share
Report
Question
Amphoteric Oxides:
Compounds of oxygen with metals or non-metals are called oxides. The metal oxides that react with both acids and bases to create salt and water or the oxides that behave as both acidic and basic oxides are known as amphoteric oxides.
These oxides undergo a neutralization reaction as they react with acid demonstrating basic property and with bases demonstrating acidic property. Elements of the groups lying towards the middle of the periodic table are generally found to be amphoteric in nature. Two such examples of Amphoteric oxides are ZnO and Al2O3. Their amphoteric nature can be seen by the following reactions:
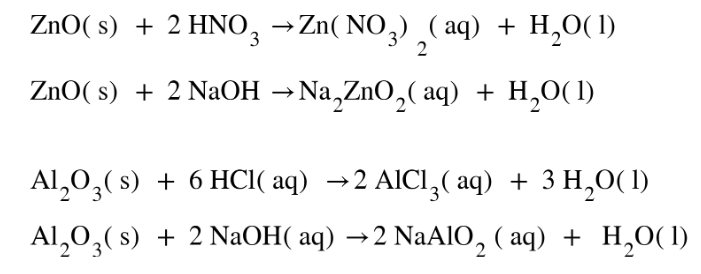
Amphoteric oxides are oxides showing both acidic and basic character. Two examples of amphoteric oxides are ZnO and Al2O3.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply