Share
What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
ReportQuestion
Allotropy refers to the occurrence of an element in multiple forms, each with the same chemical properties but differing physical properties.
Allotropes are the different forms of an element.
Diamond is the hardest known substance and has a rigid -D structure.
It is used as a cutting tool and as an abrasive.
Diamond's carbon atom is hybridised, forming strong covalent connections with four other carbon atoms.
Graphite is soft and slippery due to its layered structure, which allows different layers to slide over each other due to weak Van der Waals forces.
As a result, it is employed as a lubricant.
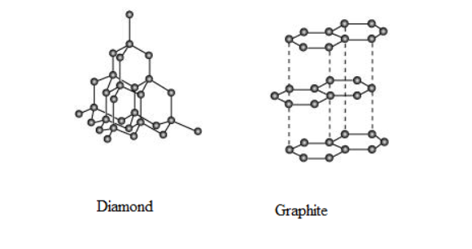
Hence, allotropes are the different forms of an element.
The -D structure of diamond make it suitable to be used as a cutting tool and as an abrasive.
The layered structure of graphite makes it suitable to be used as a lubricant.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply