Share
Report
Question
Explanation:
The Van’t Hoff factor, named after the chemist Jacobus Henricus Van’t Hoff, is the measure of the extent of association or dissociation that takes place in a solute and of how this brings about a change in the colligative properties.
The Van’t Hoff factor, represented by the symbol i, is thus given by
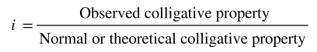
The colligative properties are those properties that depend on the concentration of the solute, such as osmotic pressure, relative lowering in vapour pressure, the elevation of boiling point and freezing point depression.
Since colligative properties are dependent on conc, they are inversely related to molecular masses.
Hence,

Final Answer:
Hence, the correct option is C: 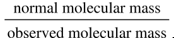
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply