Share
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas
ReportQuestion
Explanation
a) Hydrogen gas combines with nitrogen to form ammonia.
Balanced equations become;
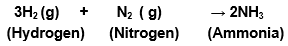
Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
Balanced equations become;
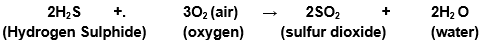
Barium chloride reacts with aluminum sulfate to give aluminum chloride and a precipitate of barium sulfate.
Balanced equations become
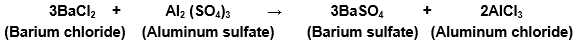
Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Balanced equations become

solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply