Share
Report
Question
Explanation:
The entropy of a system is defined as the measure of disturbance or randomness in it.
The entropy, represented by the symbol S, is given as the ratio of heat exchanged and temperature.
Mathematically, it is given as,
S = ΔQ/T
where, Δ Q is the heat absorbed and T is the temperature.
Heat exchange is a form of energy or molar energy.
Thus, to find the SI unit of entropy, we must know the SI units of heat exchange (i.e. Energy) and Temperature.
We know,
SI unit of heat exchange or molar energy or work = Joule/mole (J/mol)
SI unit of temperature = Kelvin (K)
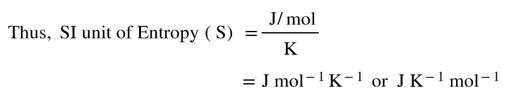
Final Answer:
Hence, the correct option is A: JK-1 mol-1
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply