Share
Report
Question
In diborane (B2H6), the Boron atoms are connected to two types of Hydrogens:
- Terminal hydrogens
- Bridging hydrogens
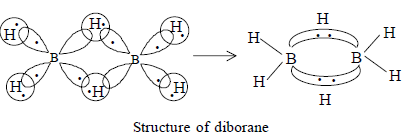
As is clear from the image, the 4 terminal hydrogens are connect to boron by covalent bonds and hence, there are four 2C - 2e- bonds
The 2 bridging hydrogens though are connected to two boron atoms each making a banana or a 3C - 2e-
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply