Share
The shape of XeF4 is
ReportQuestion
Explanation: The shape and geometry of XeF4 can be predicted by using VSEPR theory and by studying the no. of bond pairs and lone pairs. The Steric Number (S.N), which helps in predicting hybridization, can be simply calculated using the following formula:
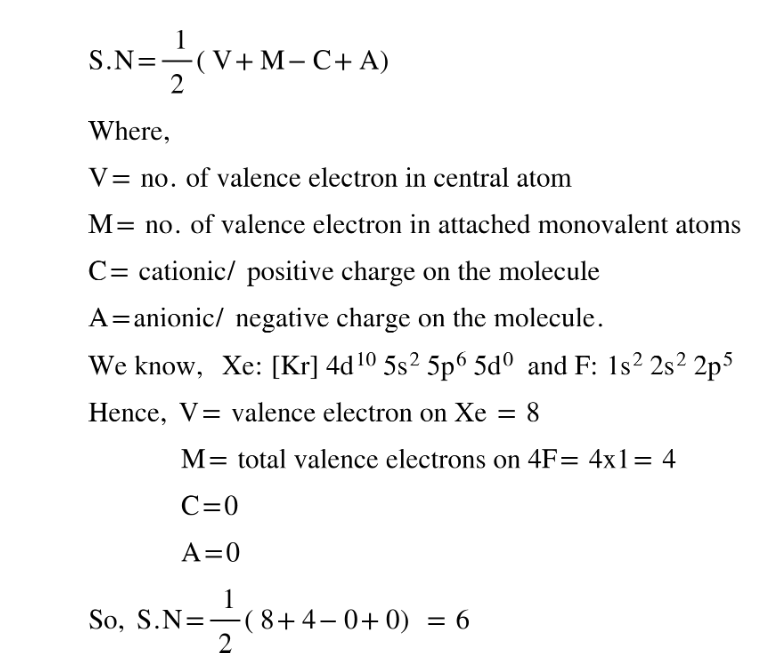
Since S.N = 6 corresponds to hybridization, the geometry of the molecule must be octahedral but the shape of the molecule depends both on bond pairs and lone pairs. Since, there are 4F attached to the central Xe atom, Bond pairs = 4
Lone pairs = (S.N- no. of bond pairs) = 6 - 4 = 2
These two lone pairs would align themselves at maximum angle so as to minimize the repulsion between themselves and hence are placed at the two opposite corners of octahedron, providing the molecule with a square planar shape.
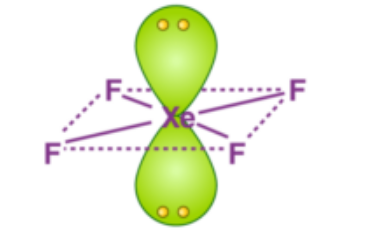
Final Answer: The correct option is C: square planar.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply