Share
The rate for the decomposition of NH3 on the platinum surface is zero order. What is the rate of production of N2 and H2 if K = 2.5 × 10-4 mol litre-1s-1
ReportQuestion
Explanation :
Given, balanced equation of decomposition of NH3 is expressed as:
2NH3 → N2 + 3H2
on the Pt metal surface is a zero order reaction.
Also given - K = 2.5 x 10-4 mol l-1s-1
where K = rate constant.
Using rate law,
rate of reaction (R) for given reaction can be written as = K [C]0 = K = 2.5 x 10-4 mol l-1s-1
Also based on rate law,
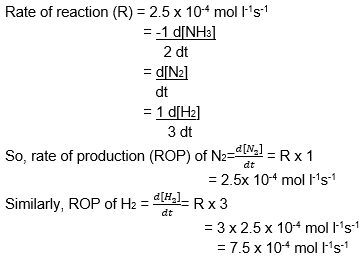
Final Answer:
Thus the ROP of N2 and H2 are 2.5 x 10-4 mol l-1s-1 and 7.5 x 10-4 mol l-1s-1 respectively.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply