Share
Question
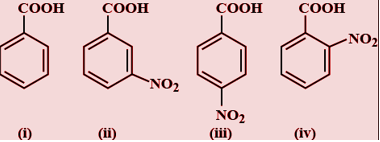
From the above groups acidity of( iv) is highest that the other because the nitro group is the strong electron withdrawing group and it also increases the acidity by removing the hydrogen ion from the carboxylate group. In iv the nitro group is present at ortho position and at this position the effect will be known as ortho effect. If nitro group will be present at p position i.e. in iii then the both resonance and inductive effect will be present. And when the nitro group is present at m-position only the inductive effect will be present here.
So the order of acidity is iv>iii>ii>i>
Hence the correct option is D)iv>iii>ii>i>
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply