Share
Report
Question
Explanation
To explain these we have to remember that P orbital has more directional nature and as orbital has non directional nature. So the P orbital has more effective overlap then that of the S orbital.
In the case of Cl2 there is an overlap between two P orbital, therefore the overlap is more effective and maximum overlap.
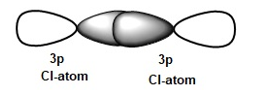
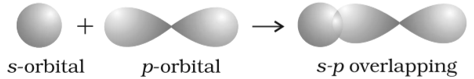
But in case of Hl, HCl, HBr the overlap between the S orbital of hydrogen and P orbital of halogen. So overlap is not that effective and minimum overlap.
Final answer
Correct Option (A)
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply