Share
Report
Question
Explanation:
According to the rules set by IUPAC for nomenclature of compounds, the given compound can be named as:
Step 1: Choose the largest chain.
Step 2: Since OH is the functional group, start the number of carbons from the end nearest to OH group.
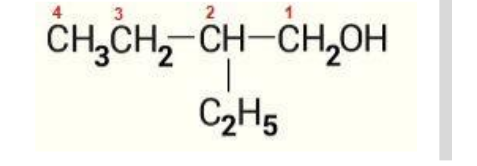
Since the given system has the longest chain with 4 carbons, the molecule is a substituted “butane”
Step 3: Designating the location of all the substituents thus, we see an ethyl group at position 2 except for the primary functionality (hydroxyl group) at 1.
Step 4: Replacing ‘e’ from alkane with ‘ol’ of alcohol.
Thus, the IUPAC name of the given compound is 2-ethylbutan-1-ol
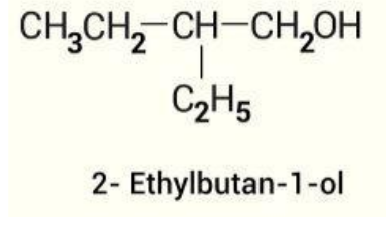
Final Answer:
Hence, the correct answer is A: 2 - ethyl - 1 - butanol.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply