Share
Report
Question
In the universe, hydrogen is the most abundant element (70% of the total mass) and it is also the principal element of the solar atmosphere.
Isotopes are elements which have the same atomic number but different mass numbers.
Hydrogen has three isotopes namely, Protium ,deuterium and tritium . The isotopes are different due to the presence of different numbers of neutrons in each of them. In protium, there are no neutrons. In deuterium we have one neutron and in tritium, we have two neutrons.
Out of these three Protium and Deuterium are stable isotopes whereas Tritium is a radioactive isotope. Tritium emits low energy particles. As the electronic configuration of isotopes is the same they have the same chemical properties but different rates of reaction.
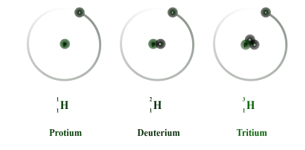
The isotopes of hydrogen are option D: Protium, deuterium and tritium
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply