Share
Report
Question
Explanation:
The enthalpy change during the formation of one mole of a compound from its constituent elements when they are in their standard state is called as standard enthalpy of formation
The reaction for Standard Molar enthalpy of formation of carbon dioxide is
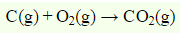
The enthalpy change when 1 mole of a compound is burnt completely in oxygen is called enthalpy of combustion so the above reaction can be regarded as combustion of carbon(graphite).
Final Answer:
The correct option is (D): The Standard Molar enthalpy of combustion of carbon (graphite).
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply