Share
(results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
ReportQuestion
Explanation: Given that at 313k, of potassium nitrate is dissolved in 100g. Thus, in 50 grms the amount of potassium nitrate needed will be
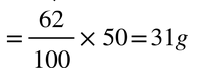
When the substance starts to cool she will observe that some soluble potassium chloride will separate in the from of crystals as the solubility of it will decrease.
Solution of salts at 293k
Potassium nitrate is 32g .
Sodium Chloride is 36g
Potassium chloride is 35g
Ammonium Chloride is 37g
Solubility of Aluminium Chloride is highest.
The solubility of salts increases with the increase in temperature.
Final Answer:
The mass of potassium nitrate is .
When the substance starts to cool she will observe that some soluble potassium chloride will separate in the from of crystals as the solubility of it will decrease.
(c) Solubility of Aluminium Chloride is highest.
(d) The solubility of salts increases with the increase in temperature.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply