Share
Report
Question
Explanation:
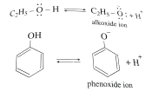
In Aqua solutions phenol release H+ ions and forms phenoxide ions. The stability of the phenoxide ion decided the acidity of the phenols in aqueous solutions
Aqua solutions of alcohol also release H+ and formed alkoxide ions.
The phenoxide ion is more stable than alkoxide ion. Reason is in phenoxide ion, the negative charge on Oxygen atoms get stabilized due to the conjugations through the benzene ring and get extra stability.
But in alkoxide ion the negative charge on Oxygen atom is localized, and doesn't go through in conjugation.
Final answer
Therefore phenol is more acidic than alcohol.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply