Share
Oxidation state of S in H2S2O8 is:
ReportQuestion
Explanation:
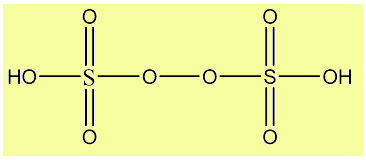
The correct answer is Option A i.e. the oxidation state of S in H2S2O8 +6.
H2S2O8 is known as Peroxydisulphiric acid. It is an inorganic compound and is also known as Marshall’s acid.
In the above figure, as it can be clearly seen that the two oxygen atoms between the two Sulphur atoms are in -1 oxidation state (peroxy linkage), and the OH group as a whole is in -1 oxidation state while all other Oxygen atoms are in -2 oxidation states. So, the sulphur in H2S2O8 has to be in +6 oxidation state.
Final Answer:
Hence, the sulphur in H2S2O8 is in +6 oxidation state.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply