Share
Report
Question
MgCl2:
The number of the valence electrons in magnesium is 2 and the number of the valence electrons in chlorine is 7.
In MgCl , total valence electrons are
In Lewis structure all the valence electrons are represented either by dots or crosses around their atoms.
Magnesium will be the central atom with two electrons and chlorine will be shown on terminal with 7 electrons each.
The Lewis structure will be
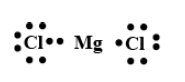
Al2O3 :
The number of the valence electrons in Aluminium is 3 and the number of the valence electrons in oxygen is 6.
In Al2O3, total valence electrons are =3+3+6+6+6=24
In Lewis structure all the valence electrons are represented either by dots or crosses around their atoms.
Each aluminium will be between two oxygens.
The Lewis structure will be
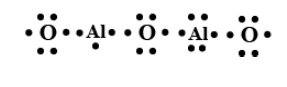
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply