Share
Report
Question
The lewis structure of SO2-4 is
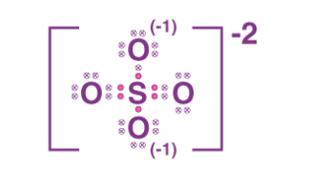
so the total valence electrons - 6+6+6+6+6+2 = 32
The lewis structure of NO-12 is
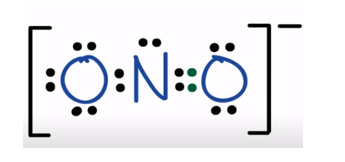
The valence electron of O is 6 and the valence electron of N is 5 .
So the total valence electron - 6+6+5+1 = 18
In lewis structure, all the valence shell electron are represented by the dots or crosses.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply