Share
Report
Question
Explanation:
It gives us insight about the partial covalent character of ionic bond.
It states that
In a ionic bond small size cation and large size anion favours covalent character in the bond.
Ionic bond have more covalent character if the charge of cation is large as polarizing power of cation increases with charge density.
For cation with same size and charge the one which have a typical transition metal electronic configuration i.e 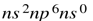 have more polarising power then the element which have nobel gas electronic configuration
have more polarising power then the element which have nobel gas electronic configuration 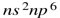 so there will be a more covalent character in the bond.
so there will be a more covalent character in the bond.
Polarising power of a cation is the ability of the cation to distort the electron cloud of anion and polarisability of anion means how easily the electron cloud get distorted.
Final answer:
We can predict the nature of a bond on the basis of Fazan rule. We can say that high polarizing power of cation and high polarisability of anion favors covalent character in the bond.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply