Share
Report
Question
When concentrated and dilute solutions are separated by a semipermeable membrane then the pressure applied on the solution side to stop the flow of solvent molecules from dilute solution to concentrated is known as osmotic pressure.
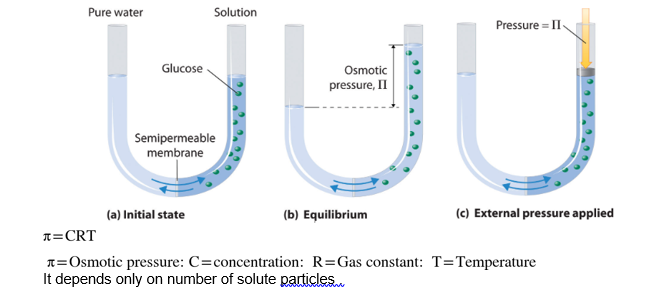
osmotic pressure is a colligative property and depends only on the number of solute particles.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply