Share
(a) Define non - metals. Give five examples of non - metals.
(b) Name a non - metal which conducts electricity.
(c) Name a non - metal having lustre (shining surface).
(d) Name a non - metal which is extremely hard.
(e) How do non - metals react with oxygen? Explain with an example. Give equation of the reaction involved. What is the nature of the product? How will you demonstrate it?
ReportQuestion
Explanation:
Non-metals are the metals which do not conduct electricity and heat.
They are non-malleable and non-ductile.
Example of non-metals are Carbon, Nitrogen, Oxygen, Fluorine, and Neon.
A non-metal which conducts electricity is Carbon.
A non-metal which is lustrous is Iodine.
A non-metal which is extremely hard is diamond which is an allotrope of Carbon.
Non-metals react with oxygen and forms acidic oxides or neutral oxides.
The example is
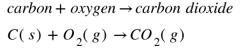
The product is acidic in nature.
It can be demonstrated as when it is dissolved in water it will form carbonic acid and will turn blue litmus to red.
Final Answer:
Non-metals are the metals which do not conduct electricity and heat. Example of non-metals are Carbon, Nitrogen, Oxygen, Fluorine, and Neon.
A non-metal which conducts electricity is Carbon.
A non-metal which is lustrous is Iodine.
A non-metal which is extremely hard is diamond which is an allotrope of Carbon.
Non-metals react with oxygen and forms acidic oxides or neutral oxides.
The example is
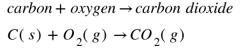
The product is acidic in nature as it forms carbonic acid when dissolve in water and turns blue litmus to red.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply