Share
Report
Question
Explanation:
The correct answer is option D which can be elaborated as follows:
Crystal field stabilization energy for an octahedral complex is given by the formula:
CFSE = (0.6 x neg - 0.4 x nt2g) ∆0
In general, splitting of octahedral complex is shown as follows:
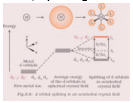
Since given octahedral complex is high spin d4, so 3 electrons occupy t2g and 1 electron occupies eg.
This splitting of high spin d4 can be diagrammatically shown as follows:
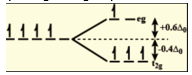
Therefore, CFSE of given complex is calculated as follows:
(0.6 x 1-0.4x 3) ∆0
= -0. 6∆0
Final Answer:
The crystal field stabilization energy (CFSE) of given high spin d4 is determined to be -0. 6 ∆0, so the correct answer is option D.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply