Share
Benzoic acid to aniline.
ReportQuestion
Explanation:
The required conversion of benzoic acid to aniline can be done in two steps, first forming benzamide, then Hoffman-bromamide degradation of the same to yield aniline.
However, earlier than conversion to benzamide, benzoic acid is transformed into benzoyl chloride,to make the addition of NH3 relatively easier considering the fact that Cl- is a good leaving group.
The steps involved in required conversion can be shown as follows:
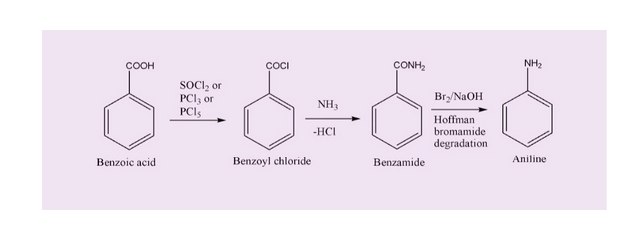
Final Answer:
Hence, the required conversion entails following steps:
i) Reaction with SOCl2 Or PCl3 to yield benzoyl chloride,ensuring better leaving group Cl- is introduced.
ii) Reaction of benzoyl chloride with ammonia to yield benzamide.
iii) Hoffman-Bromamide degradation (Br2/NaOH-reagent) to yield final product, aniline.
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply