Share
Question
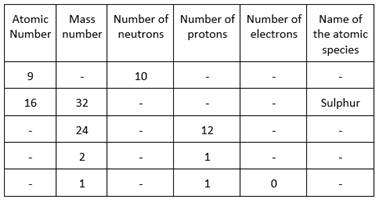
(a) First row:
(i) The given atomic number is 9. This shows that the number of protons is 9 and the number of electrons is also 9.
(ii) Mass number is equal to the sum of the number of protons and neutrons. Therefore, mass number = 9 + 10 = 19.
(iii) The atomic species having atomic number 9 is fluorine.
(b) Second row:
(i) The given atomic number is 16. This shows that the number of protons is 16 and the number of electrons is also 16.
(ii) The given mass number is 32. Number of neutrons can be calculated as , Mass number minus the number of protons. Therefore, the number of neutrons = 32 - 16 = 16
(c) Third row:
(i) The number of protons as 12. Hence,the atomic number is 12. The number of electrons is also equal to 12.
(ii) Number of neutrons can be calculated as, Mass number minus the number of protons. Therefore, the number of neutrons = 24-12 = 12.
(iii) The atomic species with atomic number 12 is magnesium.
(d) Fourth row:
(i) The number of protons is given as 1. Hence, the atomic number is 1 and the number of electrons is also equal to 1.
(ii) Number of neutrons can be calculated as, Mass number minus the number of protons. Therefore, the number of neutrons = 2-1=1.
(iii) The atomic species of atomic number 1 and mass number 2 is an isotope of hydrogen called deuterium H21 or D21.
(e) Fifth row
(i) The number of protons is given as 1. Hence,the atomic number is also 1.
(ii) The atomic species of atomic number 1 and mass number 1 is hydrogen or protium.
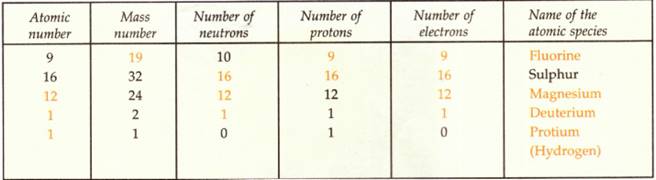
We can now write the completed Table as follows:
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply