Share
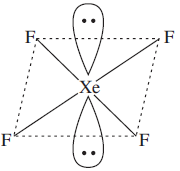
Assertion: The shape of XeF4 is square - planar
Reason: In an octahedral geometry, a single lone pair can occupy any positions but a second lone pair will occupy the opposite positions to the first lone pair.
ReportQuestion
Explanation:
XeF4 has square planar shape and octahedral geometry.
This can be elaborated as follows:
The steric number (SN) of XeF4 is 8+4/2 = 6.
So its hybridization based upon VSEPR theory is sp3d2.
The distribution of these 6 steric components could be 4 bp (bond pair) and 2lp (lone pair).
According to VSEPER repulsion between l.p- l.p > l.p - b.p . So to minimize repulsion between lone pairs they should be placed at maximum distance from each other. The maximum distance between two lone pairs in an octahedral structure(sp3d2) is possible at an angle of 180° between them i.e. the two lone pairs placed extremely opposite to each other. Hence the assertion and reason are correct and reason is the correct explanation of assertion.
Final Answer:
Hence the correct answer is option A i.e. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
solved
5
wordpress
4 mins ago
5 Answer
70 views
+22

Leave a reply