Share
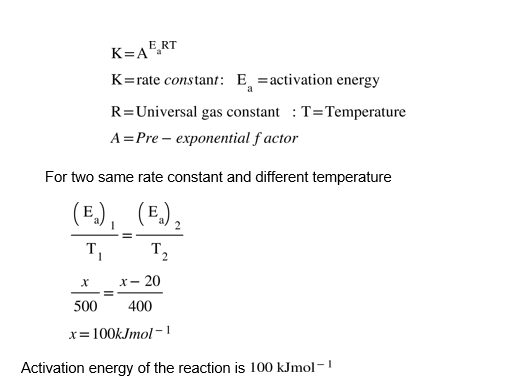
A hydrogenation reaction is carried out at 500 K . If the same reaction is carried out in presence of a catalyst at the same rate, the temperature required is 400 K . Calculate the activation energy of the reaction is the catalyst lowers the activation barrier by 20 kJ mol^-1 .
ReportQuestion

Leave a reply