Types of Chemical Reactions - Definitions Types & Explanations
Chemical Reaction
Chemical reactions are the processes in which new substances with new properties are
formed. Chemical reactions involve the breaking of bonds in the atoms of reacting substances
and making of new bonds between the atoms of products. During chemical reactions, a large
variety of rearrangement of atoms can take place to produce new substances.
Types of Chemical Reactions
Some of the important types of chemical reactions are:
1. Combination reactions
2. Decomposition reactions
3. Displacement reactions
4. Double displacement reactions, and
5. Oxidation and Reduction reactions
1. Combination Reactions: Combination reactions occur when two or more substances come together to form a single substance. For example, when magnesium combines with oxygen under heat, it forms magnesium oxide:
Example: Magnesium + Oxygen → Magnesium Oxide 2Mg + O2 → 2MgO
2. Decomposition Reactions: Decomposition reactions happen when a compound breaks down into simpler substances. This breakdown is usually triggered by heat, light, or electricity. For instance, when calcium carbonate is heated, it breaks down into calcium oxide and carbon dioxide:
Example: Calcium Carbonate → Calcium Oxide + Carbon Dioxide CaCO3 → CaO + CO2
Uses of Decomposition Reactions: Decomposition reactions are used to extract metals from their natural compounds. For example, sodium metal is extracted from molten sodium chloride by electrolysis, and aluminum metal is obtained from molten aluminum oxide through a similar process.
When we eat foods like wheat, rice, or potatoes, our body breaks down the starch into simple sugars like glucose. Proteins also break down into amino acids during digestion.
Metals vary in how they react chemically. The reactivity series sorts them from most reactive to least reactive. A metal higher in the series can replace a less reactive metal in a salt solution.
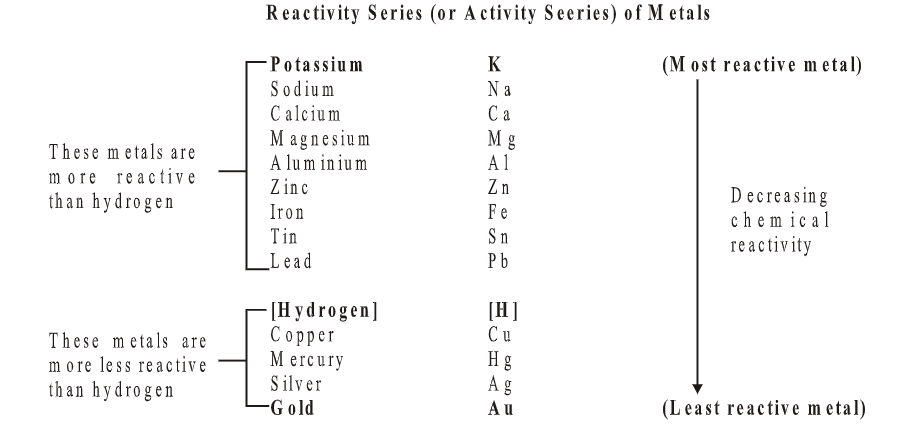
3. Displacement Reactions: Displacement reactions happen when one element replaces another in a compound. For example, when a strip of zinc is put into copper sulfate solution, it swaps places with the copper. This gives us zinc sulfate solution and copper:
Zinc (Zn) + Copper sulfate (CuSO4) → Zinc sulfate (ZnSO4) + Copper (Cu)
Zinc replaces copper because it's more reactive.
4. Double Displacement Reactions: Double displacement reactions occur when two compounds react, swapping ions to form two new compounds. For instance, if you add barium chloride to copper sulfate solution, it creates a white solid called barium sulfate, and copper chloride solution forms:
Barium chloride (BaCl2) + Copper sulfate (CuSO4) → Barium sulfate (BaSO4) + Copper chloride (CuCl2)
Here, barium chloride and copper sulfate exchange ions to produce barium sulfate and copper chloride.
- Oxidation and Reduction Reactions
Oxidation and reduction involve changes in oxygen and hydrogen in substances.
5. Oxidation:
- Adding oxygen to a substance is oxidation.
- Removing hydrogen from a substance is also oxidation.
Reduction:
- Adding hydrogen to a substance is reduction.
- Removing oxygen from a substance is also reduction.
Reduction is the opposite of oxidation. These processes often happen together.
Oxidizing Agent:
- Gives oxygen for oxidation.
- Removes hydrogen for oxidation.
Reducing Agent:
- Gives hydrogen for reduction.
- Removes oxygen for reduction.
Here are examples of oxidation and reduction reactions:
Example: When copper oxide is heated with hydrogen, copper metal and water are formed:
CuO + H2 → Cu + H2O
In this reaction:
- Hydrogen (H2) turns into water (H2O), so hydrogen is oxidized.
- Copper oxide (CuO) turns into copper (Cu), so copper oxide is reduced.
This shows how oxidation and reduction happen together in reactions.
Oxidation and Reduction of Ions
Let's understand how ions undergo oxidation and reduction using the example of molten sodium chloride in electrolysis. When electricity passes through molten sodium chloride, it breaks down into sodium metal and chlorine gas:
- Sodium chloride (molten) + Electricity → Sodium metal + Chlorine gas
This breakdown happens because of oxidation-reduction reactions. Here’s how it works based on the electronic concept of oxidation and reduction:
In molten sodium chloride (NaCl), there are sodium ions (Na+) and chloride ions (Cl–). When electricity flows through it, two main reactions occur:
-
Reduction at the Cathode:
- Positively charged sodium ions (Na+) move towards the negative electrode (cathode).
- At the cathode, they gain electrons and turn into neutral sodium atoms.
-
Oxidation at the Anode:
- Negatively charged chloride ions (Cl–) move towards the positive electrode (anode).
- At the anode, they lose electrons and become chlorine atoms.
Key Points to Remember:
- Oxidation: When an atom or ion loses electrons.
- Reduction: When an atom or ion gains electrons.
- The substance losing electrons is the reducing agent.
- The substance gaining electrons is the oxidizing agent.
Now, let’s solve a problem based on these concepts:
Example: In the compound AB, element A loses two electrons per atom, and element B gains two electrons per atom. Which element, A or B, is oxidized in the formation of AB?
Solution: Oxidation is when electrons are lost. Since element A loses electrons in forming AB, element A is oxidized.
Related Links
- Baking Soda | Preparation, Structure, Properties & Use
- Epsom salt Its Preparation & Use
- Glucose | Details About Glucose Like Its Definition, Structure & Functions
- list of amino acids | Structure of Amino Acids
- Periodic Table of Elements | HT
- Metals | What is Metals & Its Properties
- Electronegativity- Definition And Use
- Chemical Reactions – Definition & Examples
- About Washing Soda | Use And Application of Washing Soda
- What is Shielding Effect | Slater’s rules
- What is Proton | Definition, Mass , Properties & Charge of Proton
- Plaster of Paris And Gypsum Use Properties & Preparation
- What is Bleaching Powder
- What is Common Salt?
- What is Common Salt?
- Quantum Numbers | Four Types of Quantum Numbers
- Types of Chemical Reactions - Definitions Types & Explanations
- What is soil pollution?
- What is Pesticides?
- What is pH?
- What is Knocking
- What is Acid Rain?
- Definition of Suspensions
- What Is Osmosis?
- Octane Number
- NATURAL RESOURCES
- FOSSIL FUELS
- What is Ferrous sulphate?
- What is Condensation
- ADSORPTION ISOTHERM
- Cetane Number
- What is Isomerism
- Metallurgy
- What is the Reactivity Series?
- Hydrogen
- Etards Reaction
- Silicon
- Iodine
- Fluorine
- Corrosion
- Phosphorus
- Sulphur
- Chlorine
- nitrogen
- Oxygen
- Mercury
- Silver
- Lead
- Gold
- Calcium
- Copper
Frequently Asked Questions on Types of Chemical Reactions - Definitions Types & Explanations
There are four main types: synthesis (combination), decomposition, single displacement, and double displacement reactions.
The five types include synthesis, decomposition, combustion, single displacement, and double displacement reactions.
A chemical reaction is when substances change into new substances. For example, burning methane (CH₄) in oxygen (O₂) produces carbon dioxide (CO₂) and water (H₂O)
In class 10, reactions are classified into types such as combination, decomposition, displacement, and double displacement reactions based on how substances interact and change during the reaction.